This ultra-thin pacemaker is capable of resorbing itself in the body in 5 to 7 weeks. It could revolutionise the post-operative phase of heart surgery
A team of American scientists from George Washington and Northwestern Universities has just developed a new type of pacemaker that is completely resorbable once implanted. Developed some 60 years ago, this small device sends electrical impulses to the heart to regulate its beating. Conventional pacemakers usually take the form of a small metal box containing a battery and sensors that collect the biological data needed to operate the device. The major drawback is that once the battery is depleted, the patient’s chest has to be reopened to remove the device, with the attendant surgical and infectious risks. This is the advantage of the American pacemaker: much thinner than a conventional device (250 microns compared with 2 milimetres), it does not need a battery and spontaneously resorbs at the end of its life. A biodegradable pacemaker, so to speak. It consists of a simple electrode placed on the surface of the heart to deliver electrical impulses. The materials of which it is made are biocompatible and capable of dissolving in biological fluids and then evacuating through natural channels after five to seven weeks. Its electrode is controlled via an external antenna that exchanges with the stimulator using NFC (near field communication) technology, the same as for contactless bank card payments.
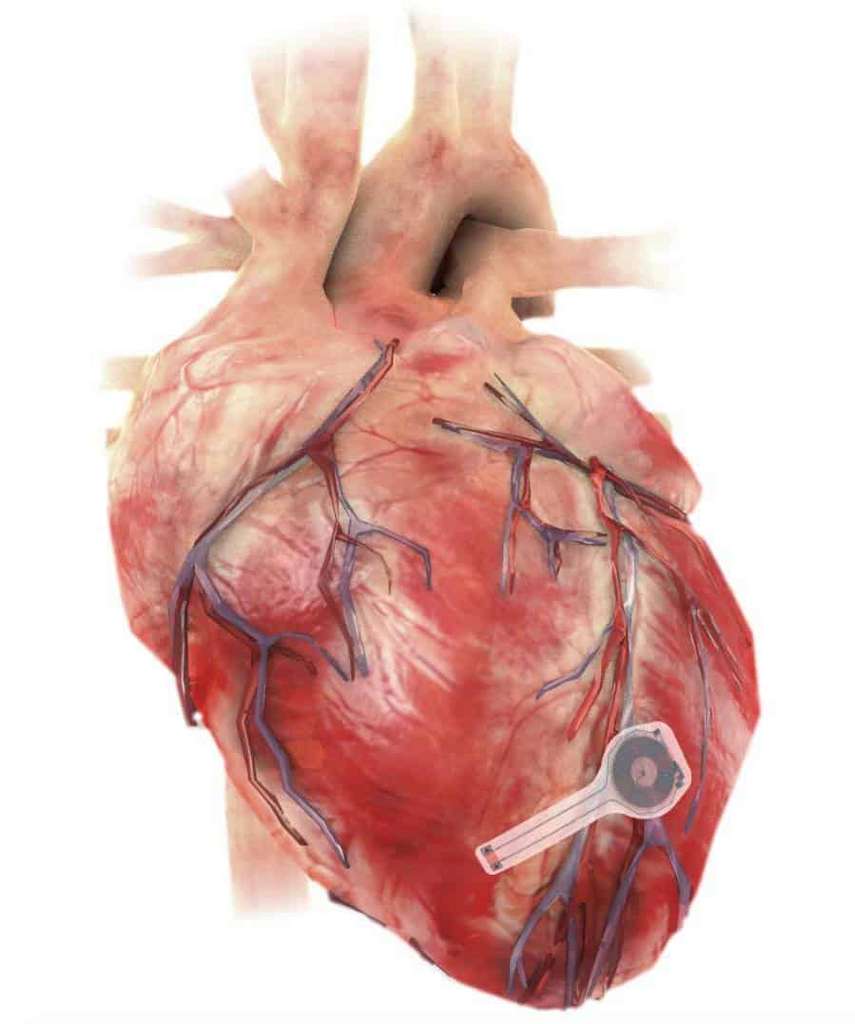
To validate its innovation, the team behind this absorbable pacemaker successfully tested it on animals before moving on to human trials. It was at the end of this last phase that the news was made public. According to them, the pacemaker can be implanted in patients who have undergone heart surgery and only need a pacemaker for a short period of time. According to John Rogers, who leads the team behind its development, “our wireless transient cardiac pacemaker overcomes the major drawbacks of traditional temporary devices, eliminating the need for percutaneous leads for surgical extraction procedures, offering the potential to reduce costs and improve patient care outcomes.”
No date has yet been announced for its approval and market launch.
innovation, #NorthwesternUniversity, #spacemaker, #cardiology, #health, #medicine,